We are currently working on:
Protein, Structure, and Function
The movement of motor proteins along microtubules (MTs) is an essential means of transportation for large cellular cargoes within the cell. MTs are linear cytoskeletal polymers composed of α/β-tubulin heterodimers associating in a head-to-tail fashion. They play crucial roles in cellular processes, such as cell division, motility, shape, and cargo transport. Molecular motors, kinesin(s), and dynein(s) are enzymatically active biological molecules that utilize the chemical energy of ATP hydrolysis to perform essential mechanical work in the cellular system. They use the MT as a platform to move cellular cargos to its minus or plus end. We are interested in the motor proteins’ functions, particularly dynein’s and the role of its heavy chain. We look at dynein from the structural bioinformatics perspective aiming to explore the mechanisms that enable regulating its motility.
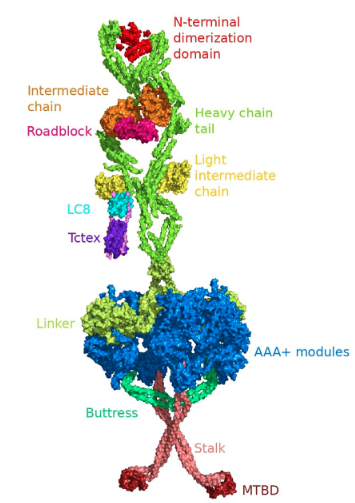
DyNEIN
Dynein-1 is a ~1.4 MDa cytoskeletal motor protein that carries organelles via retrograde transport in eukaryotic cells. The motor protein belongs to the ATPase family of proteins associated with diverse cellular activities and plays a critical role in transporting cargoes to the minus end of the microtubules. The motor domain of dynein possesses a hexameric head, where ATP hydrolysis occurs. The presented work analyzes the structure-activity relationship (SAR) of dynapyrazole A and B, as well as ciliobrevin A and D, in their various protonated states and their 46 analogues for their binding in the AAA1 subunit, the leading ATP hydrolytic site of the motor domain. This study exploits in silico methods to look at the analogues’ effects on the functionally essential subsites of the motor domain of dynein 1, since no similar experimental structural data are available. Ciliobrevin and its analogues bind to the ATP motifs of the AAA1, namely, the walker-A (W-A) or P-loop, the walker-B (W-B), and the sensor I and II. Ciliobrevin A shows a better binding affinity than its D analogue. Although the double bond in ciliobrevin A and D was expected to decrease the ligand potency, they show a better affinity to the AAA1 binding site than dynapyrazole A and B, lacking the bond. In addition, protonation of the nitrogen atom in ciliobrevin A and D, as well as dynapyrazole A and B, at the N9 site of ciliobrevin and the N7 of the latter increased their binding affinity. Exploring ciliobrevin A geometrical configuration suggests the E isomer has a superior binding profile over the Z due to binding at the critical ATP motifs. Utilizing the refined structure of the motor domain obtained through protein conformational search in this study exhibits that Arg1852 of the yeast cytoplasmic dynein could involve in the “glutamate switch” mechanism in cytoplasmic dynein 1 in lieu of the conserved Asn in AAA+ protein family.
Dynein Microtubule-binding domain
Using Molecular Dynamics (MD) simulation, Principle Component Analysis (PCA), and Normal Mode Analysis (NMA), this investigation studied large-scale movements and local interactions of dynein’s Microtubule Binding Domain (MTBD) when bound to tubulin heterodimer subunits. Examination of the interactions between the MTBD segments, and their adjustments in terms of intra- and intermolecular distances at the interfacial area with tubulin heterodimer, particularly at α-H16, β-H18, and β-tubulin C-terminal tail (CTT), was the main focus of this study. The specific intramolecular interactions, electrostatic forces, and the salt bridge residue pairs were shown to be the dominating factors in orchestrating movements of the MTBD and MT interfacial segments in the dynein’s low-high-affinity binding modes. Important interactions included β-Glu447 and β-Glu449 (CTT) with Arg3469 (MTBD-H6), Lys3472 (MTBD-H6-H7 loop) and Lys3479 (MTBD-H7); β-Glu449 with Lys3384 (MTBD-H8), Lys3386 and His3387 (MTBD-H1). The structural and precise position, orientation, and functional effects of the CTTs on the MT-MTBD, within reasonable cut-off distance for non-bonding interactions and under physiological conditions, are unavailable from previous studies. The absence of the residues in the highly flexible MT-CTTs in the experimentally solved structures is perhaps in some cases due to insufficient data from density maps, but these segments are crucial in protein binding.
Microtubule
The effects of chemotherapeutic agent vinblastine versus low temperature of 277 K were investigated on the structure of αβ-tubulin heterodimer by means of molecular dynamics simulations. Individual experiments have shown that the vinblastine-bound heterodimer, and its apo structure under low temperature of 277 K, both undergo conformational changes toward destabilization of the dimer as compared to the apo tubulin at 300 K. Both factors exhibited weakening of the longitudinal interactions of tubulin heterodimer through displacing dimer interfacial segments, resulting in dominant electrostatic repulsion at the interface of the subunits. The two independent factors of temperature and anti-mitotic agent facilitate alteration of secondary structure in functional segments such as H1-S2 loop, H3, H10 helices, and T7 loop, which are known to be important in either longitudinal or lateral contacts among αβ-heterodimers in MTs protofilaments and their depolymerization mechanism.
Drug Discovery
Our studies have shown that the toxicities of anticancer drugs and their adverse effects are related to their complex chemical structure or high molecular weight, resulting in several metabolites that could interact with off-target proteins. These factors require further attention in any drug discovery project to improve effective medical treatments, reduce the required dose of drugs, and decrease toxicities caused by the molecular complexity of medicinal agents such as antineoplastics. We work on discovering more target-selective and tolerable drugs with no or fewer adverse effects aiming to improve patients’ compliance and quality of life.
Bio-Cargo Delivery and Biomimicry
carbon nanotubes (cnts) have shown outstanding physicochemical, mechanical, electronic, and optical properties that enable a broad range of biomedical applications such as drug delivery, imaging, tissue engineering, and biosensors. their carbon hexagonal-lattice nanostructure provokes a sizeable aspect ratio and a hydrophobic surface. the latter leads to a range of limitations in cnts bioapplications, such as low solubility, aggregation, potential toxicity, and difficulties of biodegradability. functionalization of the cnts enables improving their water solubility and biocompatibility.
bIO-cARgO dELIVERY
Carbon nanotubes (CNTs) have become one of the most promising candidates for transporting drugs to target sites because of their size scale, huge surface area, and high cellular uptake. Many experimental studies of carbon nanotube drug delivery have been performed in the past decade. However, interactions with one of the essential antimitotic agents—vinblastine—and carbon nanotubes have yet to be investigated. Here we present computational studies of the interactions between vinblastine and carbon nanotubes under different conditions. We studied vinblastine–carbon nanotube interactions with one to three vinblastine molecules loaded, with armchair, chiral, and zigzag tube structures, with nonfunctionalized and ester-functionalized carbon nanotubes at 277 and 300 K. Terminal esterification of carbon nanotubes strengthened the drug–carrier interactions of all systems at 300 K. The functionalized carbon nanotubes of armchair type were suitable for drug delivery at both 277 and 300 K due to the strong drug–carrier interactions. The functionalized chiral nanotubes have been shown to be especially effective for the drug transportation at 277 K due to the enhanced drug–carrier interactions at the low temperature.
Biomimicry
a bioinspired, non-covalent carbon nanotubes (CNTs) functionalization strategy is proposed to augment their bioavailability and alleviate their biotoxicity. For functionalization, select amphiphilic peptides from a cytoskeletal biopolymer, microtubule (MT), were used. The peptides are involved in the MT polymerization by maintaining the essential lateral interactions among the MT's α- and β-tubulin subunits. They also participate in forming the MT-binding sites for hosting several MT-targeting antimitotics. Utilizing in silico methods, this study showed the peptides influenced CNT's diffusivity and aqueous solubility. The hydrodynamic shield formed by the peptides from β-tubulin was more widespread on the CNT than the α-tubulin peptides'; however, the latter created a broader hydrophobic CNT coating than those from the β-tubulin. In particular, the peptides consisting of the H1–B2, H10, H1–B2, and the M-loop, demonstrated structural features that serve to augment CNTs' water solubility and dispersibility. The performance of the peptide-functionalized CNTs as drug carriers was examined by studying seventeen antimitotics. The CNT–peptides structural composition was identified as a suitable carrier for phomopsin A, laulimalide, epothilone A, epothilone D, discodermolide, eribulin, and docetaxel. The peptides played dual roles displaying affinities to the antimitotics and the CNT; in particular, the peptides from the H1–B2 and H2–B3 loops of β-tubulin exhibited exceptional binding properties. Specific mutations on the wildtype peptides, including those from the α-tubulin M-loop and H2–B3, or the β-tubulin H1–B2, are proposed to refine their hydrophobicity, eliminate unfavorable inter-peptides electrostatic interactions or the spatial hindrance at certain regions and to enhance their conformational steadiness and exposure to the tube surface. A combination of the select amphiphilic peptides from both tubulin subunits is suggested to improve CNTs bioavailability and efficiency for carrying insoluble hydrophobic cargos.